Contributing Writer
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
Considering thermal processes for dissimilar metals
Joining steel to aluminum in heat-intensive applications
- By Jürgen Bruckner
- August 28, 2003
- Article
- Metals/Materials
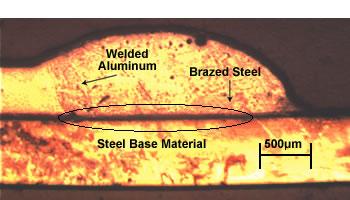 |
| This joint has dual characteristics—a brazed joint on the steel side and a welded joint on the aluminum side. |
A reliable method of joining the two most commonly used metals, steel and aluminum, would make it possible to make these joints in several applications. On one hand, aluminum is becoming increasingly more important in the automotive industry because of its good processing and performance characteristics and because it's lightweight, which results in lower fuel consumption. Given today's heightened need to decrease energy consumption, aluminum is a major player in modern-day mechanical engineering.
On the other hand, steel continues to dominate many fields of engineering, a fact underlined by the development of high-strength and superhigh-strength steels.
When different metals are joined, each metal's features should be considered. It's important to pay attention to the chemical and physical properties—such as the corrosion behavior and thermal expansion coefficient—and to atomic properties, such as the lattice constant and type of crystalline lattice.
Processes typically used to join dissimilar metals are bonding, riveting, or clinching, which essentially have no influence on the metals' atomic properties. Another method, thermal joining, can shorten joining time, produce tight seams, and provide high strength.
Thermal Joining Steel and Aluminum
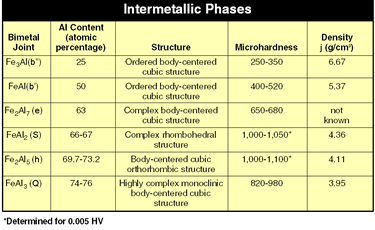 |
| Figure 1 |
Joining steel and aluminum with a high-heat-input process such as thermal joining affects the different thermal properties of the two materials—thermal expansion, heat capacity, and thermal conductivity—which may lead to very complex stress fields.
Another result of the heat input is lattice transformation and the formation of mixed phases. In iron (cubic body-centered up to 911 degrees C) and aluminum (cubic face-centered) joints, these mixed phases are characterized by high hardness and low ductility.
These intermetallic phases often have complex lattice structures and microhardness values of up to 1,000 HV or more (see Figure 1).1
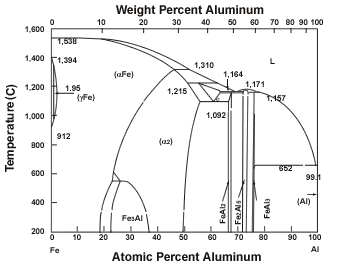 |
| Figure 2 Iron-Aluminum Phase Diagram |
The thickness of the intermetallic phases depends on the heat input rate and is the biggest problem in thermal joining. This is why all the heat-intensive processes used up until now have been designed to keep the formation of intermetallic phases within tight limits or even to prevent them from occurring in the first place.
The iron-aluminum phase diagram (see Figure 2) illustrates this problem. Only small amounts of iron can be dissolved in aluminum, and only small amounts of aluminum can be dissolved in iron. Iron and aluminum form various intermetallic phases that have low strength values (see Figure 1).
According to the model proposed by Ryabov, which shows the formation of the intermetallic phases at different temperatures, at room temperature Fe2Al5 is found on the steel side and FeAl3 on the aluminum side.2The investigations carried out in the course of our research corroborate this statement: Comparing the intermetallic phases listed in Figure 1 shows that Fe2Al5 and FeAl3 have more negative performance characteristics than the others. In particular, the Fe2Al5 phase, with its columnar crystalline structure and high hardness combined with a population density of 70 percent, has a more negative influence.
However, problems are caused not only by the processes occurring at the atomic level, but also by the differences in the metals' physical and chemical properties. Corrosion is especially important to consider. Because of the potentially large electrochemical difference of 1.22 volts between iron and aluminum (DE0), higher susceptibility to intercrystalline and galvanic corrosion must be expected. The difference between the aluminum and the zinc coating of a steel sheet (DE0) may be as much as 0.899 V.
Experiments
During our experiment in joining steel and aluminum, 1-millimeter-thick hot-dip galvanized steel sheets with a 10-micrometer zinc coating on both sides and electroplated sheets with a 7.5-µm zinc coating on both sides were joined to 1-mm-thick aluminum sheets of alloy AW 6016 using specially modified gas metal arc welding (GMAW).
 |
| Figure 3 |
The modified process was a dip-transfer arc process with special current control during metal transfer. The filler metals used were siliceous standard alloys and a basic aluminum alloy that was specially adapted for this application and was mainly intended to improve corrosion resistance.
In an initial series of tests, the sheets were joined in a lap-seam configuration, with the aluminum sheet on top. The seam length was 250 mm. The resulting joint had the characteristics of a brazed joint on the steel side and a welded joint on the aluminum side (see introductory photo).
With this process, it was possible to set the process parameters so that the intermetallic phase boundary was an average of 2 to 3 µm thick (see Figure 3).
Furthermore, this process eliminated spatter and made it possible to perform out-of-position work and to use different seam geometries, such as fillet, butt, and flanged.
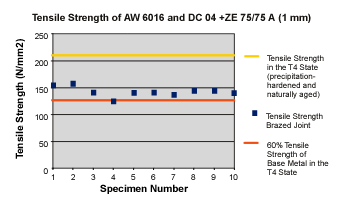 |
Initial tensile tests delivered values similar to those of single-metal aluminum joints welded with age-hardenable aluminum (seeFigure 4).
When age-hardenable alloys are welded, what occurs in the heat-affected zone (HAZ) is mainly a dehardening caused by coarse precipitations from the supersaturated mixed crystal (initial state: cold age-hardened) and a coarsening of the precipitations (initial state: artificially age-hardened).3A 30 to 40 percent decrease in tensile strength was expected. The fracture occurred along the HAZ of the aluminum every time. Initial experiments with type 5000 alloys all produced the same results—a fracture running along the HAZ.
Corrosion experiments on this experimental joint—a 120-hour salt spray test, a test in standing water, and alternating climate corrosion test—showed that when the specially modified filler metal was used, the susceptibility to corrosion was considerably reduced compared to that susceptibility in standard alloys.
Results
Bimetal joints of steel and aluminum can be produced with specially modified GMAW, using pure argon as the shielding gas. In experiments, the static strength of these joints was proven to be good. When the specimens failed, it was because of shear tension in the HAZ of the aluminum base metal.
Additionally, initial fatigue tests have shown that the strength of these joints readily bears comparison with that of welded all-aluminum joints.
Jürgen Bruckner is developing engineer and product manager with Fronius International GmbH, Buxbaumstraße 2 A-4600Wels, Austria, +43 (0)7242 241-478, fax +43 (0)7242 241394, bruckner.juergen@fronius.com, www.fronius.com.
Notes
1. D. R. G. Achar, J. Ruge, and S. Sundaresan, "Joining Aluminum and Steel, Especially by Means of Welding," Aluminum Monography, Aluminum-Verlag, Düsseldorf (1980).
2. W.R. Ryabov, Fusion Welding of Aluminum to Steel: Scientific Thoughts (Kiev: Wissenschaftliche Gedanken), 1969.
3. H. Schoer, "Welding and Brazing of Aluminum Materials," Welding and Allied Processes, DVS-Verlag, 1998.
About the Author
Related Companies
subscribe now

The Fabricator is North America's leading magazine for the metal forming and fabricating industry. The magazine delivers the news, technical articles, and case histories that enable fabricators to do their jobs more efficiently. The Fabricator has served the industry since 1970.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Trending Articles
AI, machine learning, and the future of metal fabrication

Employee ownership: The best way to ensure engagement

Steel industry reacts to Nucor’s new weekly published HRC price

Dynamic Metal blossoms with each passing year

Metal fabrication management: A guide for new supervisors

- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI